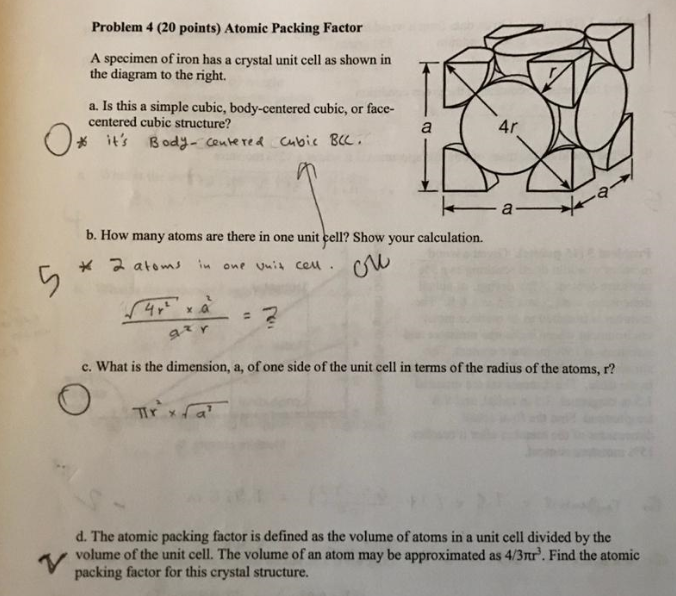
Niobium crystallizes in body-centered cubic structure. If density is 8.55 g/cm3, Calculate...... - YouTube

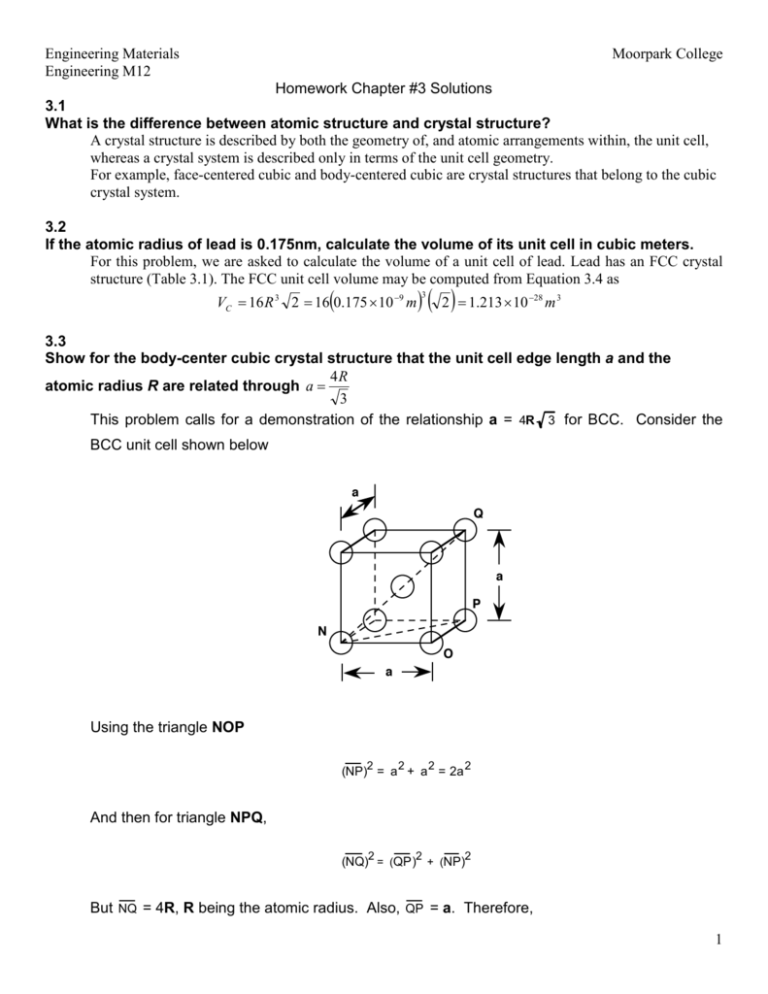
An element has a body-centered cubic (bcc) structure with a cell edge of 288pm. The density...... - YouTube

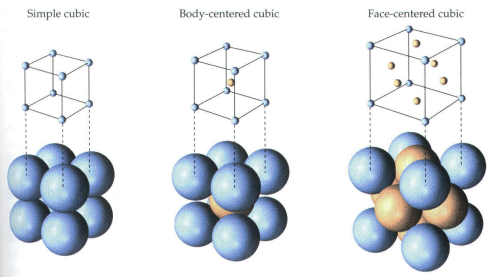
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube
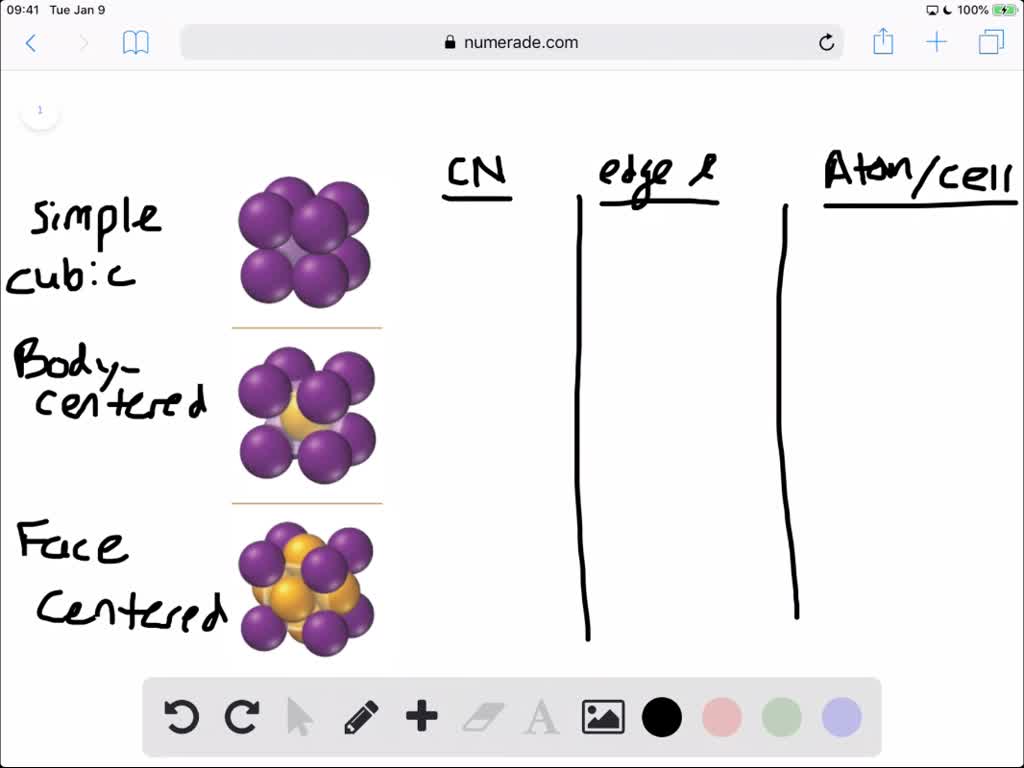
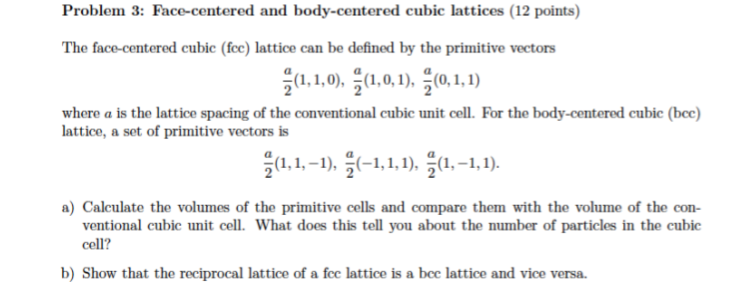
SOLVED:For each of the cubic cells in the previous problem, give the coordination number, edge length in terms of r, and number of atoms per unit cell.

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal

Unit Cell Chemistry, Atomic Radius, Density & Edge Length Calculations, Close Packed Structures - YouTube

Niobium has a density of 8.57 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a niobium atom - Chemistry Stack Exchange
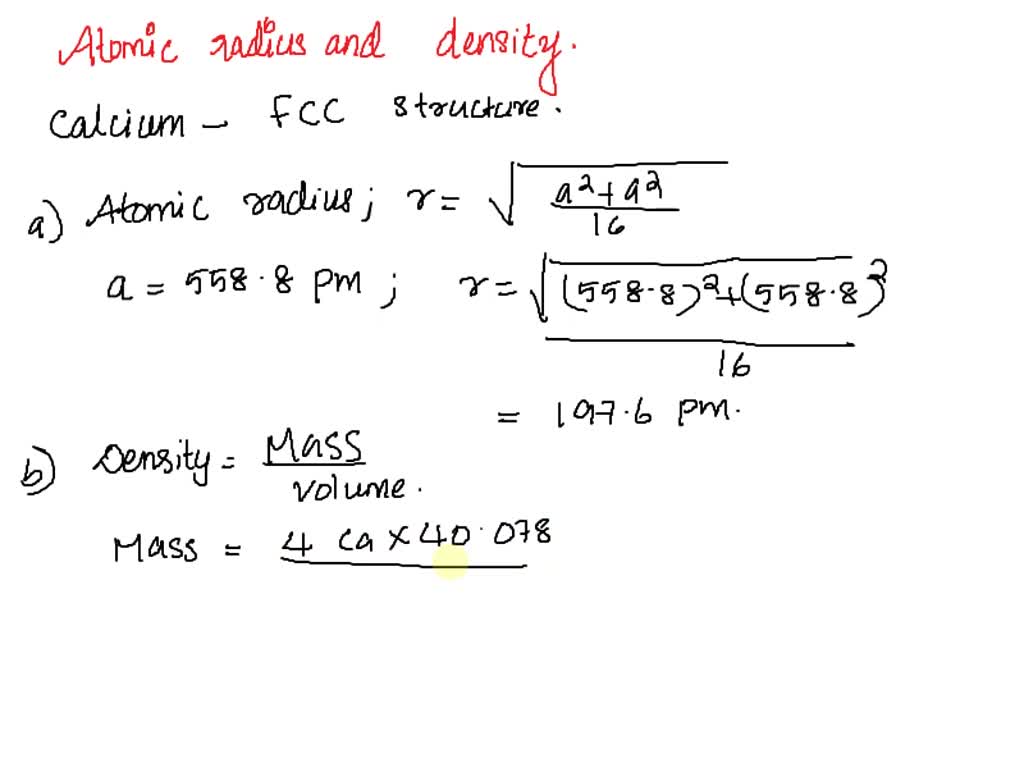
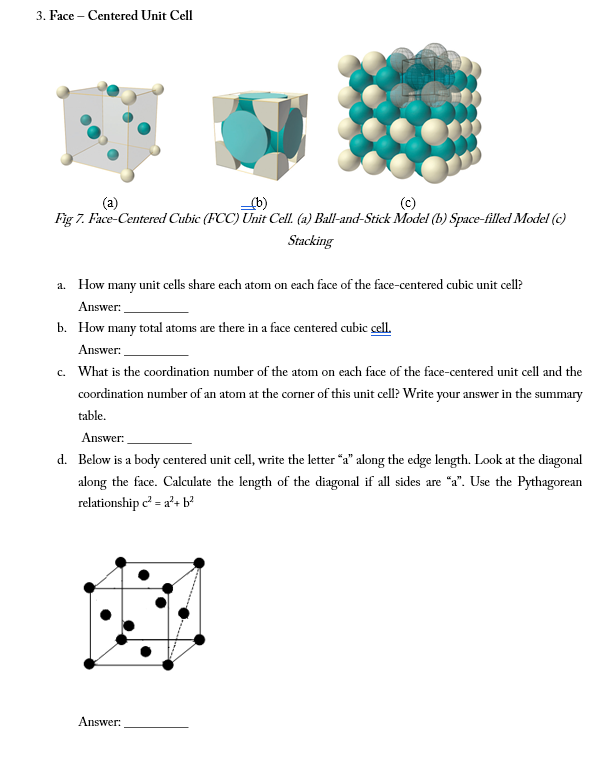
SOLVED: Calcium crystallizes in a face-centered cubic structure. The edge length of its unit cell is 558.8 pm.(a) What is the atomic radius of Ca in this structure?(b) Calculate the density of

HOW TO SOLVE THE EDGE LENGTH OF A FACE-CENTERED CUBIC (FCC) UNIT CELL | WITH PRACTICE PROBLEMS - YouTube

Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu - YouTube

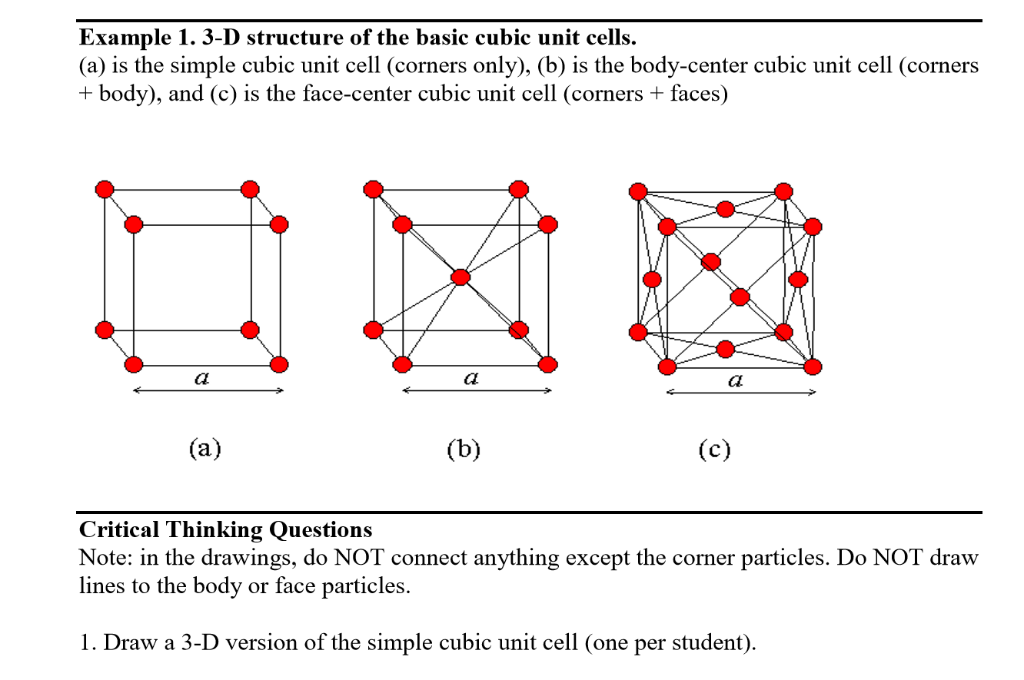

![Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com Solved 3. Face Centered Cubic Structure [10 pts] Platinum is | Chegg.com](https://media.cheggcdn.com/media/1eb/1eb9fa27-1dc7-4453-878c-a7f39a79cbf4/phppeImhN.png)